Materials Required:
- Ammeter
- Voltmeter
- Rheostat
- Beaker
- Reference electrode - Ag/AgCl
- Counter electrode - Pt electrode
- Working electrode - Lead, Zinc and Silver
- Electrolyte used - PbNO3, ZnSO4, AgNO3
Procedure:
- Choose an electrode.
- Choose an over potential.
- Choose a transfer coefficient.
- Note the current flow from the Ammeter value.
- Keeping transfer coefficient constant, find out the Current values by varying over potential.
- Find out log values corresponding to each I.
- Then plot a graph with log I vs η.
- From the intercept log I0 can be found out. Thus exchange current density I0 can be calculated for the particular system.
- Exchange current densities for different systems can be calculated.
Thus Tafel equation and Tafel Plot is verified.
| Working Electrode |
Current Density |
| Lead |
2.5 E - 13 |
| Zinc |
3 E - 11 |
| Silver |
4 E - 07 |
Observations and Calculations:
| No |
Current Density |
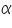 |
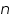 |
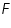 |
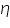 |
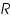 |
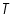 |
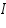 |
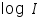 |
| 1 |
|
|
2 |
96500 |
|
8.314 |
298 |
|
|
| 2 |
|
|
2 |
96500 |
|
8.314 |
298 |
|
|
| 3 |
|
|
2 |
96500 |
|
8.314 |
298 |
|
|
| 4 |
|
|
2 |
96500 |
|
8.314 |
298 |
|
|
| 5 |
|
|
2 |
96500 |
|
8.314 |
298 |
|
|
| 6 |
|
|
2 |
96500 |
|
8.314 |
298 |
|
|
Results:
- Tafel equation and Tafel Plot is verified
- Exchange current,
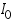 for the particular system =................
for the particular system =................
Points to Remember while Performing the Experiment in a Real Laboratory:
- Always wear lab coat and gloves when you are in the lab. When you enter the lab, switch on the exhaust fan and make sure that all the chemicals and reagents required for the experiment are available. If it is not available, prepare the reagents using the components for reagent preparation.
- Make sure to clean all your working apparatus with chromic acid and distilled water and ensure that all the apparatus are free from water droplets while performing the experiment.
- Make sure to calibrate the electronic weigh balance before taking the measurements.
- Ensure that the ammeter ,voltmeter and rheostat are working properly.
- Ensure that calomel electrode has sufficient amount of saturated KCl solution
- Clean all glass wares with soap and distilled water. Once the experiment completed recap the reagent bottles. Switch off the light, and exhaust fan before leaving the lab.
- Discard the used gloves in a waste bin.




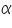
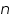
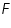
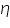
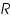
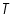
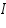
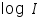
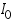 for the particular system =................
for the particular system =................