|
.
|
Procedure:
- Choose the titrant.
- Choose the titrate.
- Select the normality of the titrate.
- Choose the volume of the liquid to be pipetted out.
- Select the indicator.
- Start titration.
- End point is noted at the colour change of the solution.
- From the final reading the normality of titrant can be calculated by the equation:
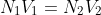
- After finding the normality, the amount of given substance in the whole of the given solution can be calculated by the equation:
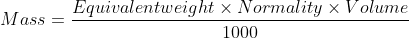
Points to Remember while Performing the Experiment in a Real Laboratory:
- Always wear lab coat and gloves when you are in the lab. When you enter the lab, switch on the exhaust fan and make sure that all the chemicals and reagents required for the experiment are available. If they are not available, prepare the reagents using the components for reagent preparation.
- Properly adjust the flame of the Bunsen burner. The proper flame is a small blue cone; it is not a large plume, nor is it orange.
- Make sure to clean all your working apparatus with chromic acid and distilled water and ensure that all the apparatus are free from water droplets while performing the experiment.
- Make sure to calibrate the electronic weigh balance before taking the measurements.
- Clean all glassware with soap and distilled water. Once the experiment is completed, recap the reagent bottles. Switch off the light, exhaust fan and gas cylinder before leaving the lab.
- Discard used gloves in a waste bin.
|
..... |



