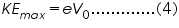| . | . | |||
| . | Photoelectric effect |
. | ||
| . | Aim:
Theory:
During his experiments on electromagnetic radiation (to demonstrate light consists of e-m waves), Hertz noticed a spark between the two metallic balls when a high frequency radiation incident on it. This is called photoelectric effect. Photoelectric effect is the emission of electrons when electromagnetic radiations having sufficient frequency incident on certain metal surfaces. We call the emitted electrons as photoelectrons and the current they constitute as photocurrent. The phenomenon was first observed by Heinrich Hertz in 1880 and explained by Albert Einstein in 1905 using Max Planck's quantum theory of light. As the first experiment which demonstrated the quantum theory of energy levels, photoelectric effect experiment is of great historical importance.
The important observations on Photoelectric effect which demand quantum theory for its explanation are:
The basic experimental set up which explains Photoelectric effect is as given below,
escape from a particular metal surface and is called work function 'W' for that metal. The work function can be expressed in terms of frequency as,
Where h is the Planck's constant and
The work function for some metals are listed in the table.
According to Einstein the Photoelectric effect should obey the equation,
From the above expression, Which says the graph connecting the maximum kinetic energy of photoelectrons 'KEmax' and frequency of incident radiation' Graph connecting 'KEmax' and frequency:
Maximum kinetic energy of photoelectrons versus frequency of incident radiation graph
Now, if we increase the reverse potential, the photocurrent gradually decreases and becomes zero at a particular reverse potential. This minimum applied reverse potential is called stopping potential V0. Hence the maximum kinetic energy of photoelectrons can be written as,
Graph connecting photocurrent and applied reverse potential :For constant intensity and different frequenciesFor constant frequency and different intensities
Cite this Simulator: |
..... | ||
| ..... | ..... | |||
 |
Copyright @ 2024 Under the NME ICT initiative of MHRD |
 |

.jpg)
 It has been observed that there must be a minimum energy needed for electrons to
It has been observed that there must be a minimum energy needed for electrons to
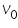 is the threshold frequency (minimum frequency for photoelectric effect).
is the threshold frequency (minimum frequency for photoelectric effect). 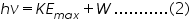

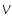 'will be a straight line with slope and Y-intercept
'will be a straight line with slope and Y-intercept 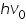 = workfunction.
= workfunction.