Materials Required:
- Calorimeter
- Thermometer
- Stirrer
Reagents:
- HCl
- NaOH
Procedure:
It is important to note that, the one should first select the sample “HCl” to proceed with the simulation. Whenever the reading on the stop watch becomes 4 minute 30 sec, the one can then select the next sample “NaOH”.
Whenever the reading of the NaOH sample becomes 4 minute 30 sec, the one can select next the Sample HCl + NaOH.
- Select HCl.
- Start the reaction by clicking “Start” button.
- Note down the time and temperature upto 4 minute 30 sec.
- Insert the values in the worksheet.
- Find out the constant temperature (T2).
- Enter the value in the worksheet.
- Select NaOH. (It will be active only after the above procedure)
- Repeat the steps 3, 4, 5 and 6 (T1).
- Select the mixture “HCl + NaOH”.
- Repeat the steps 3, 4, 5 and 6 (T3).
- Calculate the Heat of Neutralization.
Note: It is assumed that at 4 minute 30 sec the temperature of the calorimeter becomes a constant value.
Observations and Calculations:
Heat of Neutralisation
| No |
Time (sec) |
Temprature ( 0 C) |
| HCl |
NaOH |
Mixture |
| 1 |
0 |
|
|
|
| 2 |
30 |
|
|
|
| 3 |
60 |
|
|
|
| 4 |
90 |
|
|
|
| 5 |
120 |
|
|
|
| 6 |
150 |
|
|
|
| 7 |
180 |
|
|
|
| 8 |
210 |
|
|
|
| 9 |
240 |
|
|
|
| 10 |
270 |
|
|
|
| 11 |
300 |
|
|
|
| 12 |
330 |
|
|
|
| 13 |
360 |
|
|
|
| 14 |
390 |
|
|
|
| 15 |
420 |
|
|
|
| 16 |
450 |
|
|
|
| 17 |
480 |
|
|
|
| 18 |
510 |
|
|
|
| 19 |
540 |
|
|
|
| 20 |
570 |
|
|
|
Temprerature of alkali and calorimeter, 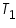 =................................................. 0 C.
=................................................. 0 C.
Temperature of acid, 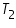 =......................................................................0 C.
=......................................................................0 C.
Temperature of mixture, 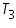 =...............................................................0 C.
=...............................................................0 C.
Water equivalent of calorimeter, W=..................................................cal.
Heat gained ,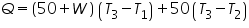 =..............................................cal.
=..............................................cal.
Heat of neutralisation=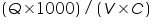 =..............................................................cal.
=..............................................................cal.
Heat of neutralisation of HCl with NaOH=....................................................................KJ.
Results:
- Heat of neutralisation of HCl with NaOH=.................KJ.
Points to Remember while Performing the Experiment in a Real Laboratory:
- Always wear lab coat and gloves when you are in the lab. When you enter the lab, switch on the exhaust fan and make sure that all the chemicals and reagents required for the experiment are available. If they are not available, prepare the reagents using the components for reagent preparation.
- Properly adjust the flame of the Bunsen burner. The proper flame is a small blue cone; it is not a large plume, nor is it orange.
- Make sure to clean all your working apparatus with chromic acid and distilled water and ensure that all the apparatus are free from water droplets while performing the experiment.
- Make sure to calibrate the electronic weigh balance before taking the measurements.
- Clean all glassware with soap and distilled water. Once the experiment is completed recap the reagent bottles. Switch off the light, exhaust fan and gas cylinder before leaving the lab.
- Discard the used gloves in a waste bin.





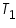 =................................................. 0 C.
=................................................. 0 C.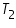 =......................................................................0 C.
=......................................................................0 C.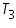 =...............................................................0 C.
=...............................................................0 C.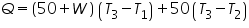 =..............................................cal.
=..............................................cal.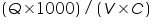 =..............................................................cal.
=..............................................................cal.