Animation flash link: https://vlab.amrita.edu/repo/CHE/SOL/Viscosity%20Measurement/index.swf
Materials Required:
- Ostwald viscometer
- Beaker
- Wash bottle
- Stop watch
- Sucker
- Pipette
- Electronic balance
- Hot Air gun
Reagents:
- Chromic acid
- Acetone
- Toluene
- Nitrobenzene
- Water
Procedure:
To determine the coefficient of viscosity of organic liquids.
- Select the liquid from the list.
- Click on the button "Fill the liquid".
- Click "Start" button. (Note: DO NOT use "Start" button on the stopwatch)
- Click "Stop" button on stopwatch when liquid reaches "label D". (Mark "Show Label" for seeing the labels)
- Note the flow time on the stopwatch.
- Calculate the Viscosity coefficient of the liquid.
| Liquid |
Mass (g) |
Density (g/cm3) |
| Water |
25.06 |
1.002 |
| Toluene |
21.55 |
0.862 |
| Nitrobenzene |
30.01 |
1.200 |
Observations and Calculations:
| Solvents |
Flow Time (sec) |
| i |
ii |
Mean |
| Water |
|
|
|
| Toluene |
|
|
|
| Nitrobenzene |
|
|
|
Room temperature = 30oC
Mass of water = 25.06 g
Mass of Toluene = 21.55 g
Mass of Nitrobenzene = 30.01 g
Time of flow of water=...........................sec
Time of flow of Toluene=......................sec
Time of flow of Nitrobenzene =............sec
Absolute viscosity of water, 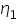 = 0.8007 centipoise
= 0.8007 centipoise
Absolute viscosity of the Toluene, 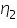 =........................centipoise
=........................centipoise
Absolute viscosity of the Nitrobenzene, 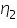 =...............centipoise
=...............centipoise
Results:
- Absolute viscosity of the Toluene = .......................centipoise
- Absolute viscosity of the Nitrobenzene =..............centipoise
Points to Remember while Performing the Experiment in a Real Laboratory:
- Always wear lab coat and gloves when you are in the lab. When you enter the lab, switch on the exhaust fan and make sure that all the chemicals and reagents required for the experiment are available. If they are not available, prepare the reagents using the components for reagent preparation.
- Make sure to clean all your working apparatus with chromic acid and distilled water and ensure that all the apparatus are free from water droplets while performing the experiment.
- Make sure to calibrate the electronic weigh balance before taking the measurements.
- Ensure that the stop watch and hot air gun are working properly.
- Clean all glassware with soap and distilled water. Once the experiment is completed recap the reagent bottles. Switch off the light, and exhaust fan before leaving the lab.
- Discard the used gloves in a waste bin.




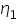 = 0.8007 centipoise
= 0.8007 centipoise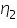 =........................centipoise
=........................centipoise