Materials Required:
- Ostwald Viscometer
- Stop Watch
- Sucker
- Pipette
Reagents:
Solvents:
- Acetonitrile
- Acetone
- Water
- Toluene
- Benzen
Polymer:
- Polyvinyl acetate
- PMMA
- Polymer Alcohol
- Polystyrene
Procedure:
Determining the Intrinsic Viscosity of the Polymer- solvent system:
- Select the Polymer.
- Select the Solvent.
- Determine the Time of flow of the solvent (t0).
- Determine the time of flow of polymer-solvent system at different concentrations.
- From the concentration and time of flow, the inherent viscosity and reduced viscosity are calculated using the equations; Inherent Viscosity =
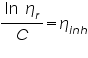 , Reduced Viscosity =
, Reduced Viscosity = 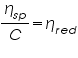
- A graph is drawn by plotting reduced viscosity against concentration and inherent viscosity against concentration.
- Intrinsic viscosity can be obtained by extrapolating the graph to zero concentration.
- From the value of intrinsic viscosity, the viscosity average molecular weight of the polymer can be calculated by using the equation.
![Double click to edit «math xmlns=¨http://www.w3.org/1998/Math/MathML¨»«mfenced close=¨]¨ open=¨[¨»«mi»§#951;«/mi»«/mfenced»«mo»=«/mo»«mi»K«/mi»«msup»«mi»M«/mi»«mi»§#945;«/mi»«/msup»«/math»](/fckeditor/editor/plugins/fckeditor_wiris/integration/showimage.php?formula=41ac099bfcfc5077fe19e713adad7ef6.png)
Observations and Calculations:
|
Conc:
(g/dl)
|
Flow Time of Polymer-Solvent system
(t) sec
|
Flow Time of Solvent
(t0) sec
|
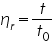 |
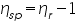 |
Reduced Viscosity,
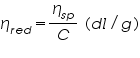
|
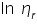 |
Inherent Viscosity,
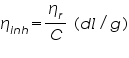
|
| 0.02 |
|
|
|
|
|
|
|
| 0.04 |
|
|
|
|
|
|
|
| 0.06 |
|
|
|
|
|
|
|
| 0.08 |
|
|
|
|
|
|
|
| 0.1 |
|
|
|
|
|
|
|
Result:
The viscosity average molecular weight of the polymer, 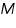 is =.......................................................
is =.......................................................
Points to Remember while Performing the Experiment in a Real Laboratory:
- Always wear lab coat and gloves when you are in the lab. When you enter the lab, switch on the exhaust fan and make sure that all the chemicals and reagents required for the experiment are available. If they are not available, prepare the reagents using the components for reagent preparation.
- Make sure to clean all your working apparatus with chromic acid and distilled water and ensure that all the apparatus are free from water droplets while performing the experiment.
- Make sure to calibrate the electronic weigh balance before taking the measurements.
- Ensure that the stop watch and hot air gun are working properly.
- Clean all glassware with soap and distilled water. Once the experiment is completed recap the reagent bottles. Switch off the light, and exhaust fan before leaving the lab.
- Discard the used gloves in a waste bin.




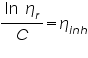 , Reduced Viscosity =
, Reduced Viscosity = 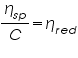
![Double click to edit «math xmlns=¨http://www.w3.org/1998/Math/MathML¨»«mfenced close=¨]¨ open=¨[¨»«mi»§#951;«/mi»«/mfenced»«mo»=«/mo»«mi»K«/mi»«msup»«mi»M«/mi»«mi»§#945;«/mi»«/msup»«/math»](/fckeditor/editor/plugins/fckeditor_wiris/integration/showimage.php?formula=41ac099bfcfc5077fe19e713adad7ef6.png)
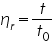
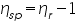
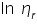
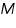 is =.......................................................
is =.......................................................