Materials Required:
- Flame Photometer.
- Cuvette.
- Gas Cylinder.
- Beaker.
- Standard flask.
- Graduated pipette.
- Wash bottle.
Reagents:
- Fruit juices: Grapes, Guava, Lime, Lemon, Mango, Orange, Papaya, Tomato, Apple.
- 5, 10 and 15 ppm Sodium and Potassium standards.
- Distilled water.
Procedure:
- Select the Test.
- Click and drag each of the standard solution below the Capillary tube and click on the "Aspirate" button to calibrate the machine.
- Drag the standard solution to place it in the orginal position.
- After calibration select the sample solution from the list.
- Click and drag the fruit juice sample below the Capillary tube and click on the " Start Test " button to measure the concentration.
Method of Preparation of Fruit Juice:
- To 10 mL of fruit juice add 50 mL deionised water.
- Filter this solution through an ashless filter paper (e.g: Whatmann 540) into a 1 liter volumetric flask.
- Make upto the mark with deionised water.
Note: If the concentration in the fruit juice are outside the range of standards the sample should be diluted accordingly.
Here the dilution factor for potassium is 100 and for sodium is 1
Observations and Calculations:
Concentration of potassium obtained from the flame photometer, 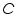 =..............................ppm.
=..............................ppm.
Therefore the concentration of potassium in the original fruit juice =  =..................................ppm.
=..................................ppm.
Concentration of sodium obtained from the flame photometer, 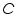 =...............................ppm.
=...............................ppm.
Therefore the concentration of sodium in the original fruit juice =  =...................................ppm.
=...................................ppm.
Results:
Concentration of potassium in the original fruit juice=..........................ppm.
Concentration of sodium in the original fruit juice=...............................ppm.
Points to Remember while Performing the Experiment in a Real Laboratory:
- Always wear lab coat and gloves when you are in the lab. When you enter the lab, switch on the exhaust fan and make sure that all the chemicals and reagents required for the experiment are available. If it is not available, prepare the reagents using the components for reagent preparation.
- Properly adjust the flame of the Bunsen burner. The proper flame is a small blue cone; it is not a large plume, nor is it orange.
- Make sure to clean all your working apparatus with chromic acid and distilled water and ensure that all the apparatus are free from water droplets while performing the experiment.
- Make sure to calibrate the electronic weigh balance before taking the measurements.
- Clean all glass wares with soap and distilled water. Once the experiment completed recap the reagent bottles. Switch off the light, exhaust fan and Gas cylinder before leaving the lab.
- Discard the used gloves in a waste bin.




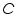 =..............................ppm.
=..............................ppm. =..................................ppm.
=..................................ppm.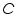 =...............................ppm.
=...............................ppm. =...................................ppm.
=...................................ppm.