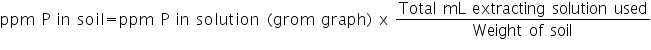Procedure:
-
Determination of Available Phosphorus in Soil:
a) Preparation of Reagents:
i. 1 N Ammonium Fluoride:
- Dissolve 37 g NH4F in distilled water and dilute the solution to 1 L.
- Store this solution in a polyethylene bottle.
ii. 0.5 N HCl:
- Dissolve 20.2 mL Con. HCl to a volume of 500 mL with distilled water.
iii. Extracting Solution:
- Add 15 mL 1N NH4F and 25 mL 0.5 N HCl to 450 mL distilled water. This gives a solution of 0.03 N NH4F and 0.025 N HCl.
iv. Dickman and Bray’s Reagent:
- Dissolve 15 g of ammonium molybdate (NH4)6Mo7O24.4H2O in 300 mL distilled water.
- Warm to about 60oC, filter and cool.
- To that add 34.2 mL of Con. HCl and make up the volume to 1 L.
- This is 1.5% solution of ammonium molybdate in the HCl. The reaction of molybdate in the HCl is as follows.
(NH4)6Mo7O24.4H2O + 6 HCl → 7 H4MoO4 + 6 NH4Cl
v. Stannous Chloride (SnCl2. 2 H2O) Solution:
- Dissolve 2 g SnCl2. 2 H2O crystals in 8.3 mL Con. HCl, dilute to 100 mL and store in a brown coloured bottle. This is 40 % SnCl2 stock solution. A piece of tin metal if added will keep the stock solution for long.
vi. Stannous Chloride Working Solution:
- Dilute 0.5 mL of the stock solution to 66 mL with distilled water. Prepare this solution just before use.
vii. Preparation of Standard Phosphorous Solution:
- 0.439 g of Potassium Dihydrogen Phosphate is weighed into a 500 ml standard flask.
- Add approximate 25 mL Con. H2SO4 to it and make up the solution.
- Pipette out 2 mL from this solution into a 10 mL standard flask and make up the solution. This gives 2 ppm stock solution of Phosphorus.
b) Extraction:
- Weigh 5 g soil and transfer it to a 100 mL conical flask.
- Add 50 mL extract solution to the soil.
- Shake the content for exact 5 minutes, and filter through Whatman No: 42 filter paper.
- Prepare a blank in which all the reagents are added similarly, except the soil.
c) Calorimetric Estimation (Dickman and Bray’s Method):
- Take 7, 25 mL standard flasks.
- Labeled one as “SAMPLE” another one as “Blank” and all others as 0.2, 0.4, 0.6, 0.8, 1.
- Pipette out 5 mL of soil extract into one 25mL standard flask which is labeled as “SAMPLE.”
- Take 5 ml “Dickman and Bray’s Reagent” using 5 ml pipette and transfer that into 25 ml standard flask which contain the Soil extract.
- To that add 7.5 mL Boric acid.
- Take the “Standard Phosphorus solution” in a clean burette.
- From this burette add 1, 2, 3 etc upto 5 mL “Standard Phosphorus solution” in previously labeled 25 mL standard flasks. (EXCEPT IN ”BLANK”).
- Pipette out 5 mL “Dickman and Bray’s Reagent” and transfer that into each 25 ml standard flask containing “Standard Phosphorus solution”.
- To that add 7.5 mL Boric acid.
- Take a test tube full of distilled water and add through the neck of the flask down to remove the adhering Ammonium Molybdate.
- Mix thoroughly the content and keep.
- Finally add 1mL SnCl2 working solution with immediate mixing and make upto the mark with distilled water once again, mix the solution thoroughly.
- Similarly prepare a blank.
- Measure the intensity of blue colour just after 10 minutes at 690 nm.
- Plot a graph between absorbance against the concentration in ppm and determine the concentration of P in soil samples from the standard curve. This is very important that colour starts fading after about 15-20 minutes at development of colour.
d) Calculation:
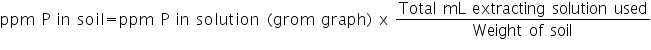
Points to Remember while Performing the Experiment in a Real Laboratory:
- Always wear lab coat and gloves when you are in the lab. When you enter the lab, switch on the exhaust fan and make sure that all the chemicals and reagents required for the experiment are available. If it is not available, prepare the reagents using the components for reagent preparation.
- Properly adjust the flame of the Bunsen burner. The proper flame is a small blue cone; it is not a large plume, nor is it orange.
- Make sure to clean all your working apparatus with chromic acid and distilled water and ensure that all the apparatus are free from water droplets while performing the experiment.
- Make sure to calibrate the electronic weigh balance before taking the measurements.
- Ensure that the desiccator has sufficient amount of desiccant; Silica gel
- Use chromic acid to clean the crucible, then heat it and make sure to cool it and before placing in the desiccators. Ensure that you are handling the crucible, with cleaned tongs or with tissue paper .Never touch it with your hand.
- Switch on the oven and adjust the temperature to 1300 C. Make sure to use a cotton glove while working with a hot air oven.
- Make sure to clean the Kipp's apparatus tube with water and ensure that it has sufficient solid material; iron sulfinide and acid, H2SO4 for producing H2S gas.
- Clean all glass wares with soap and distilled water. Once the experiment completed recap the reagent bottles. Switch off the light, exhaust fan, hot air oven and Gas cylinder before leaving the lab.
- Discard the used gloves in a waste bin.