Materials Required:
- Beaker
- Voltmeter
- Salt bridge
- Electrode Used - Li, K, Ba, Ca, Na, Mg, Al, Mn, Zn, Cr, Fe, Cd, Ti, Co, Ni, Sn, Pb, Cu, Ag, Au, 2H
- Electrolyte Used -LiCl, KCl, BaCl2, CaCl2, NaCl, MgSO4, Al(NO3)3, MnSO4, ZnSO4, Cr(NO3)3, FeSO4, CdSO4, TiNO3, CoSO4, NiSO4, SnSO4, PbNO3, CuSO4, AgNO3, AuNO3, HCl
Procedure:
- Set the temperature.
- Select the cathode from the list.
- Select the anode from the list.
- Select concentration of the electrolyte.
- Record the voltage of the cell.
- Calculate the Gibbs free energy from the voltage obtained from experiment.
- Calculate the Equilibrium constant.
- Predict the spontaneity of the cell reaction.
Observations and Calculations:
Temperature =..............................o C
Cathode used =......................
Concentration of electrolyte =...................M
Anode used =..........................
Concentration of electrolyte =...................M
Therefore, EMF of the cell =.........................V
The Gibb's free energy change of the cell reaction, 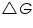 =......................
=......................
The Equilibrium constant of the cell reaction, 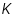 =.............................
=.............................
The spontaneity of the cell reaction =................................
Results:
- The EMF of the cell =.........................V
- The Gibb's free energy change of the cell reaction =......................
- The Equilibrium constant of the cell reaction =.............................
- The spontaneity of the cell reaction =................................
Points to Remember while Performing the Experiment in a Real Laboratory:
- Always wear lab coat and gloves when you are in the lab. When you enter the lab, switch on the exhaust fan and make sure that all the chemicals and reagents required for the experiment are available. If it is not available, prepare the reagents using the components for reagent preparation.
- Make sure to clean all your working apparatus with chromic acid and distilled water and ensure that all the apparatus are free from water droplets while performing the experiment.
- Make sure to calibrate the electronic weigh balance before taking the measurements.
- Make sure to clean the electrodes with distilled water.
- Clean all glass wares with soap and distilled water. Once the experiment completed recap the reagent bottles. Switch off the light, exhaust fan and Gas cylinder before leaving the lab.
- Discard the used gloves in a waste bin.




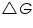 =......................
=......................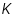 =.............................
=.............................