
| . | . | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| . | Polarography - Determination of Unknown Concentration of Vitamin C |
. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| . | Objective:
Introduction:
Jaroslav Heyrovsky received Nobel Prize in chemistry in 1959 for his discovery and development of the polarographic methods of analysis. He is known as the father of electroanalytical method. Polarographic method directly contributed to the development of innovative ideas in electrochemical techniques, instrumentation and applications.  Jaroslav Heyrovský
Theory:
Voltammetry comprises a group of electroanalytical methods widely employed in analytical chemistry and other industrial processes. In voltammetry the information about the analyte is obtained from the measurement of current as a function of applied po Polarography, is a branch of voltammetry, is the study of the electrolysis of solutions of electrooxidizable and or electroreducible substances between a dropping mercury electrode (DME) and some reference electrode (RE) which is saturated calomel electrode (SCE). This falls into the general category of linear-sweep voltammetry. In this the electrode potential is usually altered in a linear fashion from the initial potential to the final potential. The only difference in polarography is, the working electrode takes the form of a dropping mercury electrode(DME). It consists of about 10 cm of a fine capillary tubing through which mercury is forced by a mercury head of about 50cm. The diameter of the capillary tube is adjusted in such a way that a new drop will form and breaks at specific timings; usually 2 to 6s. The drop time can be controlled by a mechanical knocker that will help to dislodge the drop at a fixed time after the drop begins to form. The potential between the reference electrode and DME is varied and the resulting change in the current flow is measured. Since the linear sweep method is controlled by combined diffusion or connection mass transport method, the resulting the current versus potential response i-E curve is known as Polarogram and it is sigmoid in shape(S-Shape). The position of a wave in a polarogram along the potential axis provides an identity of the substance while the magnitude of the limiting current provides the concentration variation of this material. .jpg) Fig 1.
Since there is an increased flow of current at the starting region of the polarographic wave there is a marked decrease in the concentration of electro-active substance at the surface of the electrode. With the increase in voltage and current the concentration of the reactive species reaches to a minimal value near to the electrode surface. The current is then limited which depends on the diffusion rate of the reacting species from the solution to the electrode surface. The rapid increase of current at the final stage is due to the supporting electrolyte in the reaction. Since the concentration of the electrolyte is too large within the applied potential range, it will prevent the reactive species to reach the electrode by the electrical migration process, and hence the limiting current is assured as diffusion controlled.   Polarographic cell Tygon tube with mercury
  Reference electrode (Saturated Calomel electrode) Auxillary electrode (Platinum electrode)
Residual current is the slowly increasing current at the foot of the wave which is non faradaic in nature. The distance between the limiting diffusion plateau and the residual current is known as diffusion current (id). The potential at the midpoint of the wave is represented as half-wave potential E1/2, in this region the current is exactly half its limiting value. The limiting current is found to be the sum of the diffusion residual current. The wave height can be calculated by subtracting residual current from the limiting current. .jpg) Fig 2.
The limiting current (the plateau on the sigmoid), called the diffusion current since diffusion is the most important contribution to the flux of electroactive material at that point of the Hg drop life, is linked to analyte concentration by the Ilkovic equation: 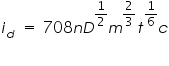 Where, id = average diffusion current during the life of the drop(µA). D= Diffusion coefficient of the analyte in the medium (cm2/s). n = Number of electrons transferred per mole of analyte. m = mass flow rate of Hg through the capillary (mg/sec). t = drop lifetime(s). c = analyte concentration (mol/cm3). 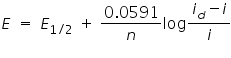 Where,
E= applied voltage. i= current. Applications:
Polarographic Analysis of Organic Compounds:
Polarography technique is employed in organic chemistry for qualitative and quantitative analysis and structure determinations of organic compounds. Since the organic compounds are insoluble in pure aqueous medium the solvent in which the organic compound and its electrode product is soluble is added to the supporting electrolyte. Examples of solvents include various alcohols or ketones, dimethyl formamide, acetonitrile, ethylene diamine and so on. The widely employed supporting electrolytes which are easily mixed with organic solvents are various quaternary ammonium salts such as tetrabutylammonium iodide. List of organic functional groups that are reducible at DME (Table 1):
Table 1.
Dibromides, Aryl halides, Alpha-halogenated ketone or aryl methane, polynuclear aromatic ring systems, and heterocyclic double bonds are capable of reducing at DME. Organic Functional Group Analysis of Non-polarographic Active Groups:
Non-polarographic active groups can be converted to into active polarographic groups and can determine its polarographic effect (Table 2).
Table 2.
Determination of Ascorbic acid (Vitamin C) in the Citrus Juice by the Standard Addition and Calibration Curve Methods:
Principle: Ascorbic acid gives a well defined polarographic oxidation wave. Use freshly prepared diluted juice for the determination of ascorbic acid.
Calibration Curve Method:
Standard Addition Methods:
Cite this Simulator: |
..... | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ..... | ..... | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
Copyright @ 2025 Under the NME ICT initiative of MHRD |
 |

 tential, under the conditions of complete concentration polarization. Voltammetry is widely used by inorganic, physical and biological chemists for nonanalytical purposes, such as the studies of the oxidation and reduction process, adsorption process in various media, and electron transfer processes at chemically modified electron surfaces.
tential, under the conditions of complete concentration polarization. Voltammetry is widely used by inorganic, physical and biological chemists for nonanalytical purposes, such as the studies of the oxidation and reduction process, adsorption process in various media, and electron transfer processes at chemically modified electron surfaces.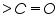
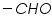
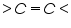
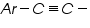
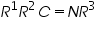
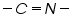
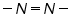
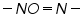
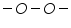
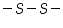
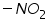
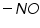
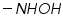
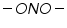
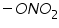
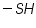 ( mercaptones oxidizes at DME and give anodic currents).
( mercaptones oxidizes at DME and give anodic currents).