|
.
|
Assignments:
-
A solution of urea in water has a boiling point of 100.130 °C. Calculate the freezing point of the same solution, 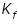 and and 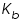 for water are -1.858 °C and 0.512 °C respectively. for water are -1.858 °C and 0.512 °C respectively.
-
A certain solution of benzoic acid in benzene freezes at 3.1 °C. What information about the number of particles and the structure of benzoic acid at the temperature can be deduced from the above data? The freezing point and cryoscopic constant, 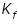 of pure benzene is 5.5 °C,-4.90 K kg mol-1 respectively. of pure benzene is 5.5 °C,-4.90 K kg mol-1 respectively.
-
Determine the molecular weight of non-volatile solute (Camphor) by cryoscopically using Carbon tetrachloride as the solvent. Kf = -29.8 (°C/(mol kg-1)), i = 1, WA = 15 g ,WB = 5 g.
-
Determine the freezing point depression constant, Kf of the solvent Carbon disulfide using Sulfur as the solute, WA = 10 g ,WB = 2 g, MB of Sulfur =256.52 g.
-
A solution of sucrose (molecular weight = 342) is prepared by dissolving 34.2 g of it in 1000 g of water. Sucrose has a van ‘t Hoff factor of, i = 1. Find out the freezing point of the solution in Kelvin. 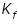 for water is -1.86 K kg mol-1. for water is -1.86 K kg mol-1.
|
..... |



