
| . | . | ||||||||||||||||||||
| . | Estimation Of Glucose |
. | |||||||||||||||||||
| . | Flash plugin EOLFlash link: https://vlab.amrita.edu/repo/CHE/ORG/Estimation_of_Glucose/index.swf
Laboratory Procedure for Estimation of Glucose:
Standardisation of Fehling’s Solution:
Prepare a solution (known standard solution) of glucose AR by weighing accurately 1.25gm and dissolving it in 250 mL standard flask in water. Make up the volume to the mark. Pipette out 20 mL each of Fehling’s A & B in a dry conical flask and shake thoroughly. Pipette out 20 mL of this freshly mixed Fehling’s solution in a clean conical flask and dilute it with 20 mL water. Heat the solution up to 70° over wire gauze. Take the standard solution of glucose prepared in a burette and run this solution slowly into the boiling Fehling’s solution until the blue colour has completely disappeared. Take care to maintain this temperature for every addition of glucose solution. Repeat the above titration by running the glucose solution steadily into the boiling Fehling’s solution until the end point is approached and then cautiously add glucose solution drop-by-drop till the end point is reached.
Alternatively to detect the end point more accurately, 5-6 drops of methylene- blue indicator may be added to the Fehling’s solution and then glucose solution added drop by drop. However, if methylene-blue is used as indicator the Fehling’s solution should not boil for more than 2-3 minutes at a stretch. The end-point here also is marked by the disappearance of the blue colour.
Simulator Procedure:
Observations and Calculations:
Normality of Titrate used, N1=.............N. Volume of Titrate used, V1 =..............mL. Volume of Titrant Used, V2 =..............mL. Therefore, the Normality of Titrant N2= The amount of substance in the whole of the given solution =
=.......................g. Result:
Points to Remember while Performing the Experiment in a Real Laboratory:
Cite this Simulator: |
..... | |||||||||||||||||||
| ..... | ..... | ||||||||||||||||||||
 |
Copyright @ 2025 Under the NME ICT initiative of MHRD |
 |

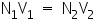
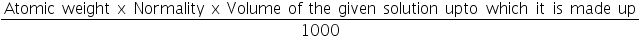
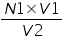 =................N.
=................N.