
| . | . | |||||||||||||||||||||
| . | Flame Photometry |
. | ||||||||||||||||||||
| . | Objective:
Introduction
Atomic spectroscopy is thought to be the oldest instrumental method for the determination of elements. These techniques are introduced in the mid of 19th Century during which Bunsen and Kirchhoff showed that the radiation emitted from the flames depends on the characteristic element present in the flame. The potential of atomic spectroscopy in both the qualitative as well as quantitative analysis were then well established. The developments in the instrumentation area led to the widespread application of atomic spectroscopy. Atomic spectroscopy is an unavoidable tool in the field of analytical chemistry. It is divided into three types which are absorption, emission, and luminescence spectroscopy. The different branches of atomic absorption spectroscopy are (1) Flame photometry or flame atomic emission spectrometry in which the species is examined in the form of atoms (2) Atomic absorption spectrophotometry, (AAS), (3) Inductively coupled plasma-atomic emission spectrometry (ICP-AES).
 Gustav Kirchhoff (left) and Robert Bunsen (right)
Theory:
Photoelectric flame photometry, a branch of atomic spectroscopy is used for inorganic chemical analysis for determining the concentration of certain metal ions such as sodium, potassium, lithium, calcium, Cesium, etc. In flame photometry the species (metal ions) used in the spectrum are in the form of atoms. The International Union of Pure and Applied Chemistry (IUPAC) Committee on Spectroscopic Nomenclature has recommended it as flame atomic emission spectrometry (FAES). The basis of flame photometric working is that, the species of alkali metals (Group 1) and alkaline earth metals (Group II) metals are dissociated due to the thermal energy provided by the flame source. Due to this thermal excitation, some of the atoms are excited to a higher energy level where they are not stable. The absorbance of light due to the electrons excitation can be measured by using the direct absorption techniques. The subsequent loss of energy will result in the movement of excited atoms to the low energy ground state with emission of some radiations, which can be visualized in the visible region of the spectrum. The absorbance of light due to the electrons excitation can be measured by using the direct absorption techniques while the emitting radiation intensity is measured using the emission techniques. The wavelength of emitted light is specific for specific elements.
Flame Photometer
Parts of a flame photometer
1. Source of flame: A burner that provides flame and can be maintained in a constant form and at a constant temperature.
2. Nebuliser and mixing chamber: Helps to transport the homogeneous solution of the substance into the flame at a steady rate.
3. Optical system (optical filter): The optical system comprises three parts: convex mirror, lens and filter. The convex mirror helps to transmit light emitted from the atoms and focus the emissions to the lens. The convex lens help to focus the light on a point called slit. The reflections from the mirror pass through the slit and reach the filters. This will isolate the wavelength to be measured from that of any other extraneous emissions. Hence it acts as interference type color filters.
4. Photo detector: Detect the emitted light and measure the intensity of radiation emitted by the flame. That is, the emitted radiation is converted to an electrical signal with the help of photo detector. The produced electrical signals are directly proportional to the intensity of light. A schematic representation of flame photometer is shown in figure 1, Fig 1: A schematic representation of flame photometer
Mechanism of working:
The working of the flame photometer involves a series of steps which is discussed in the following sections.
Nebulisation:
The solution of the substance to be analyzed is first aspirated into the burner, which is then dispersed into the flame as fine spray particles.
A brief overview of the process:
1. The solvent is first evaporated leaving fine divided solid particles.
2. This solid particles move towards the flame, where the gaseous atoms and ions are produced.
3. The ions absorb the energy from the flame and excited to high energy levels.
4. When the atoms return to the ground state radiation of the characteristic element is emitted.
5. The intensity of emitted light is related to the concentration of the element.
 Fig 2: Brief overview of the process
Events occurring in the flame:
Flame photometry employs a variety of fuels mainly air, oxygen or nitrous oxide (N2O) as oxidant. The temperature of the flame depends on fuel-oxidant ratio.
The various processes in the flame are discussed below:
The energy level diagram of the sodium atom is shown in figure 3
 Fig 3: Energy level diagram for atomic sodium
The intensity of the light emitted could be described by the Scheibe-Lomakin equation:
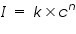 Where:
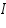 = Intensity of emitted light = Intensity of emitted light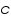 = the concentration of the element = the concentration of the element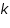 = constant of proportionality = constant of proportionality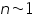 (at the linear part of the calibration curve) (at the linear part of the calibration curve)Then,
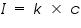 That is the intensity of emitted light is directly related to the concentration of the sample.
The comparison of emission intensities of unknown samples to either that of standard solutions (plotting calibration curve), or to those of an internal standard (standard addition method), helps in the quantitative analysis of the analyte metal in the sample solution.
The flame emissions of the alkali and alkaline earth metals in terms of the emission wavelength and the characteristic color produced by each element is shown in table 1
Applications:
Flame photometer has both quantitative and qualitative applications. Flame photometer with monochromators emits radiations of characteristic wavelengths which help to detect the presence of a particular metal in the sample. This help to determine the availability of alkali and alkaline earth metals which are critical for soil cultivation. In agriculture, the fertilizer requirement of the soil is analyzed by flame test analysis of the soil. In clinical field, Na+ and K+ ions in body fluids, muscles and heart can be determined by diluting the blood serum and aspiration into the flame. Analysis of soft drinks, fruit juices and alcoholic beverages can also be analyzed by using flame photometry.
Advantages:
Disadvantages:
Moreover the flame photometer has a wide range of applications in the analytical chemistry, it possess many disadvantages which are explained below:
Cite this Simulator: |
..... | ||||||||||||||||||||
| ..... | ..... | |||||||||||||||||||||
 |
Copyright @ 2024 Under the NME ICT initiative of MHRD |
 |







