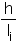| . | . | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| . | Group Theory |
. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| . |
Objective:
Introduction:
The symmetry relationships in the molecular structure provide the basis for a mathematical theory, called group theory. The mathematics of group theory is predominantly algebra. Since all molecules are certain geometrical entities, the group theory dealing with such molecules is also called as the ‘algebra of geometry’.
Symmetry Element:
A symmetry element is a geometrical entity such as a point, a line or a plane about which an inversion a rotation or a reflection is carried out in order to obtain an equivalent orientation.
Symmetry Operation:
A symmetry operation is a movement such as an inversion about a point, a rotation about a line or a reflection about a plane in order to get an equivalent orientation.
The various symmetry elements and symmetry operations are listed in below table.
Centre of symmetry:A point in the molecule from which lines drawn to opposite directions will meet similar points at exactly same distance. Some of the molecules, which have a centre of symmetry, are: N2F2, PtCl4, C2H6
1,2-di chloro-1,2-di bromoethane(all trans and staggered)
Plane of symmetry:
A plane which divides the molecule into two equal halves such that one half is the exact mirror image of the other half. The molecules, which have plane of symmetry, are: H2O, N2F2, C2H4
The broken line in the σ- plane. If we look from left side (A) into the mirror plane, HA appears to have gone on the other side and its image appears exactly at HB. Similarly viewing the structure of H2O molecule from the right side (B), the reflection of HB appears at HA configuration II is the result of this reflection operation and is equivalent to I. Another round of this operation on the molecule (configuration II) yields configuration III which is identical to configuration I.
Proper Axis of symmetry:
An axis passing through the molecule about which when the molecule is rotated 360/n an equivalent orientation is produced. This is an axis of n-fold symmetry or an axis of order as shown below. H2O, N2H3, BF3
Initially, H2O molecule is in configuration I, lying flat on the plane of the paper , and after rotating it through an angle θ = 1800 about an axis passing through O atom (Z-axis) and having HOH angle, the configuration II will be obtained. The configuration II is equivalent to configuration I, but not identical. By another similar rotation about Z-axis on configuration II, the molecule goes into configuration III. Here configuration III is identical to the initial or original configuration I.
Principle Axis:
If there is more than one axis of symmetry, in many cases one of the axes is identified as principal axis, which will be selected in the following order:
The principal axis is taken as the vertical axis that is in the z-direction. The subsidiary axis is perpendicular to the principal axis and will, hence, be in the horizontal direction.
(a Cn axis can combine with only n C2 axis perpendicular to it or with no subsidiary axis.)
The plane of symmetry is also classified on the basis of the principal axis. The planes including or involving the principal axis are called vertical planes (σv) and the planes perpendicular to the principal axis is called horizontal plane (σh).
Improper Axis of symmetry:
An axis passing through the object about which when the object is rotated through 360/n followed by reflection in a plane perpendicular to the axis produces an equivalent orientation.
For example ethane molecule (staggered form).
Configuration I and II are not equivalent i.e., θ = 600 and the consequence C6 – rotational operation is not a valid symmetry operation by itself. Similarly, II and III are not equivalent, thus showing that σ operation perpendicular to the so called C6 rotational axis is also not a genuine symmetry operation. But the configurations I and III are equivalent, so that C6 followed by σ perpendicular to C6 is a genuine, through the combined operation this product operation results in an element called S6 axis.
Identity Element:
This element is obtained by an operation called identity operation. After this operation, the molecule remains as such. This situation can be visualized by two ways. Either
Every molecule has this element of symmetry and it co-exists with the identity of the molecule, hence the name identity element.
Point group:
The symmetry elements can combine only in a limited number of ways and these combinations are called the point groups.
Nomenclature of the point group:
Identification of molecular point groups:
The whole molecules are divided into three broad categories.
Molecules of Low Symmetry (MLS):
The starting point could be the molecules containing no symmetry elements other than E, such molecules are unsymmetrically substituted and these molecules are said to be belongs to C1 point group.
The TeCl2Br2 molecules with its structure in gaseous phase belongs to C1 point group, and tetrahedral carbon and silicon compounds of the formula AHFClBr (A=C,Si).
Molecules of High Symmetry (MHS):
In this category all the molecules containing Cn axis (invariably in the absence or presence of several other types of symmetry of elements) are considered. There are three main types of point groups Cn, Dn, and Sn.
Cn type point group:
The molecules which contain only one Cn, proper axis are considered. The presence of Cn implies the presence of (n-1) distinct symmetry elements whether n is even or odd. Since Cn generates a set of n elements including E, the order of this group is n, (h=n) the molecules belonging this group are designated as Cn point groups.
This group contains a Cn axis and n σv planes of symmetry. When n is odd, all the planes are σv type only, and if n is even, there are n/2 planes of σv type and another n/2 planes of σv’ type
This set of point group can by adding a horizontal plane (σh) to a proper rotational axis, Cn. This group has a total of 2n elements –n elements from Cn and other n elements can be generated by a combination of Cn and σh, leading to the corresponding Sn axes. When n is even, Cnh point group molecules necessarily contains a centre of inversion, i.
S-trans-1,3-Butadiene - C2h Boric acid - C3h Dn type point groups:
These are purely rotational groups that are they contain only rotational axis of symmetry. When the molecule containing only one type Cn axis, it was classified as Cn point group. if in addition to one the Cn axis, a set of n Cn axes perpendicular to Cn are added, it belongs to another point group called Dn point group. The order, h, of this rotational group is 2n, since Cn generates (n-1)+E elements and the number of C2s are n more.
For example gauche or skew form of ethane contains D3 point group.
Biphenyl (skew) - D2
This point group can be obtained by adding a horizontal (σh) plane to a set of Dn group elements. The order of this Dnh group is 4n. In addition to the n elements of Cn when n is even, the elements generated are quite distinct and different from what has already been obtained. However when n is odd, we get set of n elements based on Sn axis.
Example is - B2H6 - D2h
This point group can be obtained by adding a set of dihedral planes (nσd) to a set of Dn group elements. This would thus require that there is a Cn proper axis along with nC2 s perpendicular to Cn axis and nσd planes, constituting a total of 3n elements thus far.
Example is - Cyclohexane (chair form) - D3d
Sn type point groups:
Sn axis is the only group generator for the Sn (n= even) point group of molecules. The point groups Cnh, Dnh, and Dnd .when n is odd, the presence of Sn axis implies the presence of 2n elements, in which a plane of symmetry (σ) makes an independent appearance. Thus the presence of a plane perpendicular to Cn or Sn axis and other additional elements would lead to the other point group such as Cnh , Dnh ,or Dnd when n is even and there is no plane perpendicular (σh ) to this axis, the presence of other elements in addition to Sn axis leads to only Dnd point group.
Example is - SiO4(CH3)4 - S4
Point Groups and their Detailed List of Symmetry Elements are Included in the Below Table.
Molecules of special Symmetry:
This class has two groups of molecules:
In addition to all the linear molecules, circle-shaped and cone-shaped ones also belong to this category. These can be further sub-divided into two groups, C∞v and D∞v groups, the presence or absence of i used to distinguish between these two types of groups.
This group can be defined the same way as that of Cnv group, where n is infinity. The C∞ axis lies along the inter nuclear molecules, and since the molecule is linear the σv planes are infinite in number. The order of this group is h = ∞. All hetero nuclear molecules, and all unsymmetrically substituted linear polyatomic molecules are belongs to this point group.
Examples are HX (X = F, Cl, Br, I), CO, NO, CN etc.
This group is an extension of Dnh group (∞). This group of molecules contain a C∞ axis, ∞C2 axes perpendicular to C∞ axis and a σh plane. Then, it would also imply that the molecule possess ∞σv planes and a centre of inversion(i). So all centre of symmetric molecules are belongs to this point group.
Homo nuclear diatomic molecules such as N2, O2 , H2 , F2 and Cl2 etc.
Molecules Containing Multiple Higher-Order Axes:
This is a special class of molecules which contain more than one type of rotational axes (n≥2) that are neither perpendicular to the principal Cn axis (n-highest), as in Dn and related point groups, nor bear any perpendicular relationship. These high-symmetry molecules have shapes corresponding to the five platonic solids: tetrahedral, octahedral, cube, dodecahedral and icosahedra.
Tetrahedral Point Groups:
The highest-fold axis in these point groups is C3 axis, which is occur in multiples. Molecules with only C3 axes and additionally only C2 axes belong to T, a pure rotational point group, since they contain only proper rotational axes. All other type of elements (σv ,i, Sn) are absent in three groups. T: 8C3 (4C31, 4C32), 3C 2, E Si(CH3)4 When σd, S4 (collinear with C2 axes) elements are added to the T group elements, we get a full group called Td. The order of this group is 24.
Td: 8C3 (4C31, 4C32), 3C 2, E, 6S4 (S41, S43), 6 σd
CCl4
There is another uncommon point group, Th, which can be obtained by adding three planes of symmetry (σh) to T group. The order of this is group is 24.
Th :8C3 (4C31, 4C32), 3C 2, i, 3σh, 8S6 (4S61, 4S65)
Example - Co(NO2)63-
Octahedral Point Groups:
This is another class of cubic groups. Additionally, octahedral point groups have multiple C4 axes when compared to that of tetrahedral groups.
When the group contains only rotational axes, it is labelled as O group, h, of this group are 24.
O: E, 6C4 (3C41, 3C42), 8C3 (4C31, 4C32), 6C 2, 3C 2 ‘=3C42
To the O group elements, if 3σh and 6 σd planes are added, a group of higher symmetry can be generated. The order of this group is 48.
Oh E, 6C4 (3C41, 3C42), 3C 2 ‘=3C42, 6C 2, 8C3 (4C31, 4C32), i, 3σh, 6σd, 6S4 (S41, S43), 8S6 (4S61, 4S65)
Cubane
Icosahedral Groups:
This group contains molecules with either icosahedral or pentagonal dodecahedral shapes and belongs to Ih point groups. The molecules containing only the rotational elements are said to be belongs to I point group. The order of this point group is 60, whereas that full group is 120.
I E, 24C5 (6C51, 6C52, 6C53, 6C54), 20C3 (10C31, 10C32), 15C 2 Ih E, 24C5, 20C3, 15C 2,24S10 (6S101, 6S1036S107,6S109), 20S6 (10S61, 10S65), i, 15σ
Fullerene
Great Orthogonality Theorem:
The matrices of the different Irreducible Representations (IR) possess certain well defined interrelationships and properties. Orthogonality theorem is concerned with the elements of the matrices which constitute the IR of a group. The mathematical statement of this theorem is,
Where,
i, j – Irreducible Representations
li, lj – Its dimensions
h – Order of a group
Γi(R)mn – Element of mth row, nth column of an ith representation
Γj(R)'m'n' - Element of m' th row, n' th column of j' th representation
δij δmm' δnn' – Kronecker delta
Kronecker delta can have values 0 and 1. Depending on that the main theorem can be made into three similar equations.
i.e., 1. When, Γi ≠ Γj and j ≠ i, then δij = 0
Therefore, ΣR [ Γi(R)mn ] [ Γj(R)'m'n' ]* = 0
2. When, Γi = Γj and j = i, then δij = 1
Therefore, ΣR [ Γi(R)mn ] [ Γi(R)'m'n' ]* = 0
From these two equations we can say the Orthogonality theorem as, “the sum of the product of the irreducible representation is equal to zero”.
3. When i = j, m = m', n = n'
Then, ΣR [ Γi(R)mn ] [ Γi(R) mn]* =
From the above equations some important rules of the irreducible representations of a group and there character were obtained.
Five Rules Obtained:
1. The sum of the squares of the dimensions of the representation = the order (h) of the group.
i.e., Σli2 = l12 + l22 + l32 + …… ln2 = h
Γi(E) –the character of the representation of E in the ith IR which is equal to the dimension of the representation.
i.e., Σi [ Γi(E)]2 = h
2. The sum of the squares of the characters in any IR is equal to ‘h’.
i.e., ΣR [ Γi(R)]2 = h
3. The vectors whose components are the characters of two different IR are orthogonal.
i.e., ΣR Γi(R) Γj(R) = 0 when i ≠ j.
4. In a given representation (reducible/irreducible) the characters of all matrices belonging to operations in the same class are identical.
Eg:- in C3v point group there are, E, 2C3, 3 σv. there characters are same for a particular IR.
5. No: of irreducible representation in a group = No: of classes in a group.
Applications:
Applying these 5 rules we can develop the character table for various point groups. For most chemical applications, it is sufficient to know only the characters of the each of the symmetry classes of a group.
Steps for The Construction of A Character Table::
Character Table for C2v Point Group:
1. For C2v point group, there are 4 symmetry operations, Γ1, Γ2, Γ3, Γ4 therefore, it contains 4 classes. i.e., E, C2z, σxz, σyz. And character of E is denoted as l1, l2, l3, l4.
2. The sum of the squares of the dimensions of the symmetry operations = 4.
i.e., l12 + l22 + l32 + l42 = h = 4.
This can only be satisfied by four one dimensional representations.
The unknowns for Γ1 is a1, b1, c1 , for Γ2 is a2, b2, c2.
3. Sum of the squares of the characters of any IR is equal to the order of the group.
i.e., 12 + a12 + b12 + c12 = 4.
4. The orthogonality theorem must be satisfied by all the symmetry operations.
i.e., ΣR Γi(R) Γj(R) = 0 i.e., for Γ1 . Γ2 i.e., 1.1 + a1 .1 + b2 . 1 + c2 .1 = 0 Let a2 = 1, b2 = -1 and c2 = -1 Then Γ1 . Γ2 = 0
For Γ3 . Γ1 i.e., 1.1 + a3 .1 + b3 . 1 + c3 .1 = 0 Let a3 = -1, b3 = 1 and c3 = -1 Then Γ1 . Γ2 = 0
For Γ4 . Γ1 i.e., 1.1 + a4 .1 + b4 . 1 + c4 .1 = 0 Let a4 = -1, b4 = -1 and c4 = 1 Then Γ1 . Γ2 = 0
Rules For Assigning Mullicon Symbols:
1. If the IR is unidimensional term A or B is used. If it is two dimensional E is used. If it is three dimensional T is used.
2. If one dimensional IR is symmetric with respect to the principle axis Cn, i.e., character of Cn is +1, the term A is used. If it is -1, the term B is used.
3. If IR is symmetric with respect to subsidiary axes then subscript 1 is given and is antisymmetric then subscript 2 is given.
4. Prime and double prime marks are used for indicating symmetric or antisymmetric with respect to horizontal plane.
5. ‘g’ and ‘u’ subscripts are given for those which are symmetric and antisymmetric respectively with respect to centre of symmetry then,
In any character table there are 4 different areas.
Area I – Characters of symmetry operations Area II – Mullicon Symbols Area III – Cartesion coordinates of rotation axes. Area IV – Binary Products
Area III:
In order to assign the cartesion coordinates, different operations are performed on each of the axes. Here we find the symbols X, Y, Z represents coordinates and rotations Rx, Ry and Rz.
Consider a vector along with Z axes, the identity doesn’t change the direction of the head of the vector. On doing C2, σxz, σyz operations no change will occur. Hence its characters are 1 1 1 1. Therefore the vector ‘Z’ transforms under A1.
Similarly,
The characters are 1 -1 1 -1 corresponding to B1. And with respect to vector Y, 1 -1 -1 1 and therefore corresponds to B2. Similar arrangement could be made to rotation axes Rx, Ry, Rz representing rotation about XZ axes. In order to see how they transformed, a curved arrow should be considered around the axes. If the direction of the head of the curved arrow doesn’t change due to operation, the character is +1, otherways it is -1.
The characters are 1 1 -1 -1. Therefore it will be A2 and it becomes Rz.
The characters are 1 -1 1 -1. Therefore it will be B1 and it becomes Rx. Similarly B2 become Ry.
Therefore,
Area IV:
Which represents the squares and binary products.
A1 = Z = 1 1 1 1 A12 = Z2 = 1 1 1 1 = A1 B1 = X = 1 -1 1 -1 B12 = X2 = 1 1 1 1 = A1 B2 = Y = 1 -1 -1 1 B22 = Y2 = 1 1 1 1 = A1 XY = B1 . B2 = 1 1 -1 -1 = A2 XZ = B1 . A1 = 1 -1 1 -1 = B1 YZ = B2 . A1 = 1 -1 -1 1 = B2
Therefore the actual character table for C2v point group will be,
Character Table for C3v Point Group:
1. For C3v point group, there are 6 symmetry operations and 3 classes, i.e., Γ1, Γ2, Γ3.
2. The sum of the squares of the dimensions of the symmetry operations = 6.
i.e., l12 + l22 + l32 = h = 6.
This can only be satisfied by, 2 one dimensional and 1 two dimensional representations.
3. The sum of the dimensions of Γ1 also 6.
Therefore, its characters are (1 1 1).
4. All operations must satisfy the orthogonality condition, ΣR Γi (R) Γj (R) = 0
i.e., For Γ1 . Γ2 i.e., 1.1 + 2 . a2 .1 + 3 . b2 . 1 = 0 Let a2 = 1 and b2 = -1 Then Γ1 . Γ2 = 0
i.e., For Γ3 . Γ2 i.e., 2.1 + 2 . a3 .1 - 3 . b3 . 1 = 0 Let a3 = -1 and b3 = 0 Then Γ3 . Γ2 = 0
For any character table there are 4 areas.
For Area I:
Assign the Mullicon symbols.
For Area III:
In order to assign the Cartesian coordinates different operations are performed on each of the axes. Here we were finding the symbols X, Y, Z represents coordinates and rotations Rx, Ry and Rz.
Consider,
The characters are 1 1 1 corresponding to A1.
The characters are 1 -1 1, the character corresponding to C3 will be -1. Therefore it will be E. Similarly for vector Y, we get 1 -1 1 and this also E.
Similar arrangement could be made to rotation axes Rx, Ry, Rz.
The characters are 1 -1 1. Therefore it corresponds to E and it will become Rx.
The characters are 1 1 -1. Therefore it corresponds to A2 and it will become Rz.
Similarly for E the characters are 2 -1 0 and it will become Ry.
Area IV:
Which represents the squares and binary products.
A1 = Z = 1 1 1 A12 = Z2 = 1 1 1 = A1 XY = E = 2 -1 0 = E XZ = E . A1 = 2 -1 0 = E YZ = E . A1 = 2 -1 0 = E
Therefore the actual character table for C3v point group will be,
Some Important Character Tables for Molecular Point Groups:
Cite this Simulator: |
..... | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ..... | ..... | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
Copyright @ 2024 Under the NME ICT initiative of MHRD |
 |


















![Double click to edit «math xmlns=¨http://www.w3.org/1998/Math/MathML¨»«msub»«mi mathvariant=¨normal¨»§#931;«/mi»«mi mathvariant=¨normal¨»R«/mi»«/msub»«mo»§nbsp;«/mo»«mo»[«/mo»«msub»«mi mathvariant=¨normal¨»§#915;«/mi»«mi mathvariant=¨normal¨»i«/mi»«/msub»«mo»(«/mo»«mi mathvariant=¨normal¨»R«/mi»«msub»«mo»)«/mo»«mrow»«mi mathvariant=¨normal¨»m«/mi»«mi mathvariant=¨normal¨»n«/mi»«/mrow»«/msub»«mo»]«/mo»«mo»§nbsp;«/mo»«mo»[«/mo»«msub»«mi mathvariant=¨normal¨»§#915;«/mi»«mi mathvariant=¨normal¨»j«/mi»«/msub»«mo»(«/mo»«mi mathvariant=¨normal¨»R«/mi»«msup»«mo»)«/mo»«mo»§apos;«/mo»«/msup»«msup»«mi mathvariant=¨normal¨»m«/mi»«mo»§apos;«/mo»«/msup»«msup»«mi mathvariant=¨normal¨»n«/mi»«mo»§apos;«/mo»«/msup»«msup»«mo»]«/mo»«mo»*«/mo»«/msup»«mo»§nbsp;«/mo»«mo»=«/mo»«mo»§nbsp;«/mo»«mfrac»«mi»h«/mi»«mrow»«msqrt»«msub»«mi»l«/mi»«mi»i«/mi»«/msub»«/msqrt»«msub»«mi»l«/mi»«mi»j«/mi»«/msub»«/mrow»«/mfrac»«mo»§nbsp;«/mo»«msub»«mi mathvariant=¨normal¨»§#948;«/mi»«mrow»«mi mathvariant=¨normal¨»i«/mi»«mi mathvariant=¨normal¨»j«/mi»«/mrow»«/msub»«mo»§nbsp;«/mo»«msub»«mi mathvariant=¨normal¨»§#948;«/mi»«mrow»«mi mathvariant=¨normal¨»m«/mi»«msup»«mi mathvariant=¨normal¨»m«/mi»«mo»§apos;«/mo»«/msup»«/mrow»«/msub»«mo»§nbsp;«/mo»«msub»«mi mathvariant=¨normal¨»§#948;«/mi»«mrow»«mi mathvariant=¨normal¨»n«/mi»«msup»«mi mathvariant=¨normal¨»n«/mi»«mo»§apos;«/mo»«/msup»«/mrow»«/msub»«/math»](/fckeditor/editor/plugins/fckeditor_wiris/integration/showimage.php?formula=f99c74d531e3a7e6728d28eb1c55327e.png)