Materials Required:
- Magnetic stirrer
- Stop watch
- Ammeter
- Voltmeter
- Rheostat
- Switch
- Alkaline Battery
- Beaker
- Pt gauze cathode
- Pt spiral anode
- Electrolytes Used -CuSO4,PbSO4,MnSO4,NiSO4,CdSO4
Procedure:
- Select the solution.
- Choose the cathode.
- Choose the anode.
- Select the voltage.
- Select the resistance.
- Select the time.
- Then click on the start button.
- Note the total weight of cathode.
- From that the mass of metal deposited should be determined.
Observations and Calculations:
Initial weight of the cathode = 10 g
Final weight of the cathode =.......................g
Therefore, the amount of metal deposited, 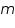 =...................g
=...................g
Voltage used =....................................V
Resistance used =............................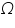
Therefore, the Current, 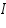 =.................A
=.................A
Time taken for compete deposition, 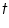 =.....................sec
=.....................sec
Result:
The amount of metal deposited on the electrode =.....................g
Points to Remember while Performing the Experiment in a Real Laboratory:
- Always wear lab coat and gloves when you are in the lab. When you enter the lab, switch on the exhaust fan and make sure that all the chemicals and reagents required for the experiment are available. If it is not available, prepare the reagents using the components for reagent preparation.
- Make sure to clean all your working apparatus with chromic acid and distilled water and ensure that all the apparatus are free from water droplets while performing the experiment.
- Make sure to calibrate the electronic weigh balance before taking the measurements.
- Ensure that the voltmeter, ammeter and rheostat are working properly.
- Clean all glass wares with soap and distilled water. Once the experiment completed recap the reagent bottles. Switch off the light, exhaust fan and Gas cylinder before leaving the lab.
- Discard the used gloves in a waste bin.




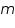 =...................g
=...................g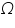
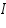 =.................A
=.................A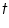 =.....................sec
=.....................sec